Lateral Flow Assays (LFAs) have emerged as crucial diagnostic tools, particularly in the realm of blood testing. These assays provide a rapid and simple method for detecting the presence or absence of specific analytes in blood samples, making them invaluable in various healthcare settings. In this article, we will delve into the fundamentals of Lateral Flow Assays, exploring their structure, function, and the manufacturing processes that drive their effectiveness.
What is a Lateral Flow Assay?
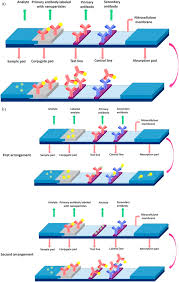
A Lateral Flow Assay is a paper-based diagnostic device designed to detect the presence of a target substance in a liquid sample, such as blood. The assay consists of several key components, including a sample pad, conjugate pad, nitrocellulose membrane, and an absorbent pad. The sample is applied to the sample pad, initiating a capillary flow that transports the sample across the different components of the assay.
How Does Lateral Flow Assay Work?
The heart of a Lateral Flow Assay lies in its ability to produce visible results within minutes. The sample, often a small amount of blood, moves through the device via capillary action. At the conjugate pad, specific molecules (antibodies or antigens) linked to colloidal gold or other markers react with the target analyte, forming a complex.
As the sample progresses, this complex encounters the test line on the nitrocellulose membrane, where a second set of antibodies or antigens capture the complex, generating a visible line. If the target analyte is present, the test line will appear, indicating a positive result. Additionally, a control line further down the membrane confirms that the test has functioned correctly.
Lateral Flow Manufacture: The Key to Reliable Assays
The manufacturing process of Lateral Flow Assays plays a pivotal role in their effectiveness. It involves precise placement of reagents, controlling the flow dynamics, and ensuring the consistency of the components. Lateral Flow Manufacture employs advanced techniques to achieve reproducibility and reliability in large-scale production.
Manufacturers carefully select materials, optimize pad configurations, and validate production processes to guarantee the consistent performance of each assay. Quality control measures are implemented to minimize variability, ensuring that the end product meets the stringent standards required for accurate and dependable results.
Applications and Advantages
Lateral Flow Assays find application in various medical fields, ranging from infectious disease diagnosis to pregnancy testing. Their simplicity, rapid results, and cost-effectiveness make them ideal for point-of-care testing and resource-limited settings. These assays have proven instrumental in the early detection of diseases, enabling timely interventions and improving patient outcomes.
Advancements in Lateral Flow Technology
Recent advancements in lateral flow technology have expanded the capabilities of these assays, enhancing their sensitivity and specificity. Researchers and manufacturers continue to explore novel materials, such as nanoparticles and advanced biomarkers, to improve detection limits and broaden the range of applications. These innovations contribute to the ongoing evolution of lateral flow assays, positioning them as versatile tools in the diagnostic arsenal.
Challenges and Considerations
While Lateral Flow Assays offer numerous advantages, they are not without challenges. Sensitivity can be a limitation, especially in cases where low concentrations of the target analyte need to be detected. Ongoing research focuses on overcoming these challenges by refining assay designs, exploring new detection strategies, and harnessing the power of digital technologies to augment traditional lateral flow techniques.
Future Prospects and Integration
The future of Lateral Flow Assays holds exciting prospects, particularly in the integration with digital technologies. The marriage of lateral flow with smartphone apps and connected devices allows for real-time data analysis, remote monitoring, and seamless integration into healthcare systems. This convergence of traditional diagnostics with digital solutions enhances accessibility and data-driven decision-making.
Environmental Impact and Sustainability
As the global focus on sustainability intensifies, the environmental impact of diagnostic technologies comes under scrutiny. Lateral Flow Manufacturers are exploring eco-friendly materials and manufacturing processes to reduce the environmental footprint of these assays. The development of biodegradable components and sustainable packaging aligns with the growing emphasis on environmentally conscious practices in healthcare.
Regulatory Landscape
The regulatory landscape surrounding diagnostic assays, including lateral flow devices, is dynamic. Stringent quality control measures and adherence to regulatory guidelines are paramount to ensure the safety and reliability of these devices. Manufacturers navigate evolving regulatory requirements to bring innovative lateral flow assays to market, balancing speed to market with compliance.
Conclusion
In conclusion, Lateral Flow Assays for blood have evolved into sophisticated diagnostic tools, offering rapid results and versatility in various healthcare applications. The ongoing integration of advanced technologies, consideration of environmental impact, and addressing challenges in sensitivity contribute to the continual enhancement of these assays. As they navigate the dynamic landscape of diagnostics, Lateral Flow Manufacturers play a crucial role in shaping the future of point-of-care testing and decentralized healthcare solutions.